
Suche

PRIORITY PROGRAM
“Porous Metal-Organic
Frameworks”
 Coordinator & Contact
Coordinator & Contact
 Summary
Summary
 Projects and
Projects andParticipants - Part I
 Projects and
Projects andParticipants - Part II
EVENTS
 Topical Workshop 2009
Topical Workshop 2009MOF Modelling for PhD
students
 Kickoff meeting 2009
Kickoff meeting 2009
 Topical Workshop 2010
Topical Workshop 2010Adsorption and Diffusion
in MOFs for PhD
students
 MOF 2010 - Marseille
MOF 2010 - Marseille Meeting after MOF 2010 -
Meeting after MOF 2010 - Marseille
 Workshop "MOF Synthesis and
Workshop "MOF Synthesis andStructure London 2010"
 2. Assessment SPP 1362
2. Assessment SPP 1362 in Dresden 2011
 Topical Workshop "Catalysis"
Topical Workshop "Catalysis"for PhD students Stuttgart
2011
 International Symposium on
International Symposium on
Metal-Organic Frameworks
2011 in Dresden
 Workshop "MOFs for industrial
Workshop "MOFs for industrial
applications Bergamo 2011"
 Topical Workshop "MOF-Based
Topical Workshop "MOF-Based
Chemical Sensors" München
2012
 MOF Status Report Meeting
MOF Status Report Meeting
2012 - Dresden
 International MOF Symposium
International MOF Symposium
2013 - Dresden
LINKS
 Chair of Inorganic Chemistry I
Chair of Inorganic Chemistry I
MOF Based Sorption Sensors by Rare Earth Luminescence
Consortium: |
Professor Dr. Claus Feldmann, Karlsruhe
|
Dr. Ralf Köhn, Hamburg
|
|
Prof. Dr. Klaus Müller-Buschbaum, Würzburg
|
|
Project: |
MOF Based Sorption Sensors by Rare Earth Luminescence |
Abstract: |
The project entitled „MOF Based Sorption Sensors by Rare Earth Luminescence” combines luminescence as an intrinsic property of rare earth elements with sorption abilities of MOFs. The combination of both will be used to develop a new type of sensors for adsorption processes by influencing and quenching of the luminescence. The project consists of two different approaches: In the first instance luminescence as an intrinsic property of the MOF itself by using lanthanide metal centres is used on the base of Ln-NMOFs which are already known for their luminescence. In contrast to carboxylate frameworks amides are much more endurable vs. energy sources and thus also vs. the excitation radiation. The investigation of the framework luminescence depending on different adsorbents like NH3, N2O, NO, NO2, CO2, CO, SO2, SO3, H2, O2 etc and improved MOFs is planned. The second approach of the project is the utilization of known carboxylate MOFs such as IRMOF-8 or MIL-82 and to study luminescent rare earth particles, both in solutions as well as solid particles in dispersions and their interaction with the MOF structures. This expands the project to non-inert conditions and aqueous solutions. Altogether the main goal is an application of suitable sensors that allow determining sorption by changes or quenching of the luminescence, the latter being a luminescence switch. In order to investigate chemically different adsorbents development of suitable surface determination methods is planned. Therefore the project combines expertises of Ln-Amide and MOF chemistry, luminescence as well as sorption and surface determinations. |
Results: |
 |
 |
|
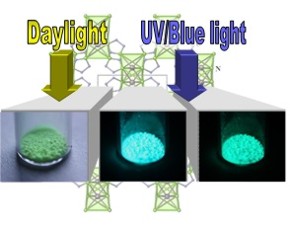
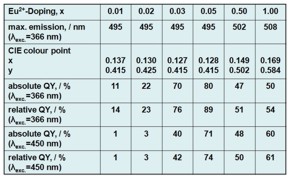
Figure 1 and Table 1: The luminescent MOF 38[Sr1-xEux(Im)2] (x = 0.01-1.0) excited by UV and visible light as well as data on the excitation, emission, colour point and quantum efficiency. The project takes a successful course regarding the evaluation of luminescence and porosity determinations of Ln-N-MOFs, the syntheses of new and improved MOFs regarding regarding both properties. Promising substances for a sensor effect of the dependence of luminescence and sorption have been found. Two MOFs show luminescence in combination with a selective adsorption of gases. In both cases adsorption of CO2 results in quenching of the luminescence so that the principle goal of a sorption sensor by rare earth luminescence was reached. Moreover the MOF with highest quantum efficiency (almost 90%) of all MOFs known today and dye-modified zirconium phosphates (DMZP’s) as an alternative class of luminescent nano particles for MOF incorporation were synthesized. |
Publications: |
A. Zurawski, M. Mai, D. Baumann, C. Feldmann, K. Müller-Buschbaum
|
|
J.-C. Rybak, C. J. Höller, P. R. Matthes, K. Müller-Buschbaum, M. Mai, C. Feldmann, R. Köhn
|
|
J.-C. Rybak, I. Schellenberg, R. Pöttgen, K. Müller-Buschbaum
|
|
A. Zurawski, F. Hintze, K. Müller-Buschbaum
|
|
C. J. Höller, K. Müller-Buschbaum
|
|
C. J. Höller, M. Mai, C. Feldmann, K. Müller-Buschbaum
|
|
C. J. Höller, P. Matthes, J. Beckmann, K. Müller-Buschbaum
|
|
J.-C. Rybak, M. Tegel, D. Johrendt and K. Müller-Buschbaum
|
|
A. Zurawski, J. Sieler, K. Müller-Buschbaum
|
|
J.-C. Rybak, K. Müller-Buschbaum
|
|
J.-C. Rybak, Y. Mokaddem, K. Müller-Buschbaum
|
|
K. Müller-Buschbaum, Y. Mokaddem
|
|
K. Müller-Buschbaum, Y. Mokaddem
|
|
M. Roming, H. Lünsdorf, K. E. J. Dittmar, C. Feldmann
|
|
M. Roming, H. Lünsdorf, K. E. J. Dittmar, C. Feldmann
|
|
H. Goesmann, C. Feldmann
|
|
H. Goesmann, C. Feldmann
|
|
M. Roming, C. Feldmann
|
|
M. Roming, C. Feldmann
|
OTHER EVENTS
- Calendar of events TU Dresden
- 16.-17.09.2013 (Dresden, GERMANY)
International MOF Symposium 2013 - 08.-11.09.2014 (Leipzig, GERMANY)
FEZA2014 - 28.09.-01.10.2014 (Kobe, JAPAN)
MOF2014 - Starting on 18.04.2014 (Hamburg, GERMANY)
Crystals & Symmetry Course
PUBLICATION NEWS
- TU Dresden’s DUT-6 claimed by other scientists
- 10.05.2012
Themed Issue on Metal-Organic Frameworks now published - 05.12.2012
Deuterium from a quantum sieve
CONTACT
Project Assistant
![]()
![]()
Phone: +49 351 463-33632
Fax: +49 351 463-37287
Email:
sekretariat-ac1@mailbox.tu-dresden.de
Office:
![]()
Bergstraße 66,
Neubau Chemische Institute,
Zi. 462
Mail to:
![]()
TU Dresden
Fachrichtung Chemie
und Lebensmittelchemie
![]()
Professur für Anorganische Chemie I
![]()
01062 Dresden
Bulk mail to:
![]()
Fachrichtung Chemie
und Lebensmittelchemie
![]()
Professur für Anorganische Chemie I
![]()
Helmholtzstraße 10
01069 Dresden