
Suche

PRIORITY PROGRAM
“Porous Metal-Organic
Frameworks”
 Coordinator & Contact
Coordinator & Contact
 Summary
Summary
 Projects and
Projects andParticipants - Part I
 Projects and
Projects andParticipants - Part II
EVENTS
 Topical Workshop 2009
Topical Workshop 2009MOF Modelling for PhD
students
 Kickoff meeting 2009
Kickoff meeting 2009
 Topical Workshop 2010
Topical Workshop 2010Adsorption and Diffusion
in MOFs for PhD
students
 MOF 2010 - Marseille
MOF 2010 - Marseille Meeting after MOF 2010 -
Meeting after MOF 2010 - Marseille
 Workshop "MOF Synthesis and
Workshop "MOF Synthesis andStructure London 2010"
 2. Assessment SPP 1362
2. Assessment SPP 1362 in Dresden 2011
 Topical Workshop "Catalysis"
Topical Workshop "Catalysis"for PhD students Stuttgart
2011
 International Symposium on
International Symposium on
Metal-Organic Frameworks
2011 in Dresden
 Workshop "MOFs for industrial
Workshop "MOFs for industrial
applications Bergamo 2011"
 Topical Workshop "MOF-Based
Topical Workshop "MOF-Based
Chemical Sensors" München
2012
 MOF Status Report Meeting
MOF Status Report Meeting
2012 - Dresden
 International MOF Symposium
International MOF Symposium
2013 - Dresden
LINKS
 Chair of Inorganic Chemistry I
Chair of Inorganic Chemistry I
MOF Based Sorption Sensors by Rare Earth Luminescence
Consortium: |
Dr. Florian Beuerle, Würzburg
|
Professor Dr. Claus Feldmann, Karlsruhe |
|
Professor Dr. Klaus Müller-Buschbaum, Würzburg |
|
Project: |
MOF Based Sorption Sensors by Rare Earth Luminescence |
Abstract: |
The project focuses on a combination of two properties: luminescence and sorption, with their depen-dency rendering a new sensoring for sorption accessible via observation of an emission. In the prelim-inary period it was successfully shown that luminescence can be implemented into Ln-N-MOFs as an intrinsic property leading to the MOFs with the highest quantum efficiencies known today. It was also proven that microporosity is observed in combination with luminescence in the same MOF material and that luminescence can be quenched by certain adsorbants. To improve the selectivity, new MOFs with linkers suitable both as antennas as well as modifiers of the pore systems are aims of the second period. Novel inverse MOFs based on functionalized fullerenes are included in which the organic linker constitutes the connectivity centers interlinked by metal ions. This offers two perspectives: Intrinsic luminescence with improved quenching options upon sorption as well as the inclusion of luminescent nanoparticles into the inverse MOF. Functionalized fullerenes offer nanometer spacing from their own extensions in contrast to conventional MOFs and ZIFs that do not incorporate these particles. Lumi-nescence will be characterized by photoluminescence spectroscopy, quantum yield, decay determina-tions, and porosity utilizing BET and drifting balances for pressures up to 200 bar. Quantification of the dependency luminescence versus sorption to evaluate the sensor effect by simultaneous fluorescence / porosity studies is the final goal of the project. |
Results: |
The MOF system 2∞[Ln2Cl6(bipy)3]·2bipy can be successfully used for an efficient tuning of the emission colour. During MOF formation different ratios of Eu3+ and Tb3+ can be implemented into the MOF by mixing their trichlorides with GdCl3 under solvent free melt conditions of 4,4’-bipyridine, as the three trivalent lanthanides form a series of mixed crystal MOFs. Depending on the ratio of the three lanthanides networks of the formula 2∞[Gd2-x-yEuxTbyCl6(bipy)3]·2bipy, 0.1 ≤ x,y ≤ 0.5 are formed. 4,4’-bipyridine functions as efficient antenna. A perfect series of luminescence tuned hybrid materials can be formed that covers the complete visible spectrum in-between green and red emission including yellow and orange. As Gd3+ is not involved in the radiative processes it can be utilized as a matrix to dilute the two other lanthanide ions within a majority of gadolinium connectivity centers. The materials show multifunctionality as the luminescence is retained during activation of the MOFs for microporous materials. |
|
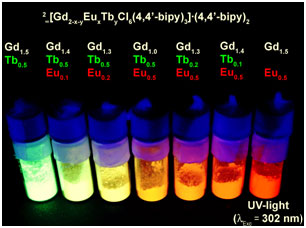 Photoluminescence of the series of solid solutions 2∞[Gd2-x-yEuxTbyCl6(bipy)3]·2bipy under UV-light (? = 302 nm), 2-8 being arranged in uprising number from left to right. |
|
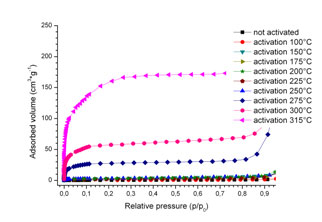 N2 adsorption isotherms at 77 K for 2∞[Gd2Cl6(bipy)3] in cm3g-1 uptake depending on variation of the activation temperature of the MOF. |
|
Aiming at sensor-type features based on nanoparticle-modified MOFs, inorganic-organic hybrids such as the compound ZrO(FMN) (FMN: flavin mononucleotide) have been introduced as novel luminescent nanomaterials. ZrO(FMN) comprises several important benefits, including a quick and easy water-based synthesis, potentially low costs of production, a high biocompatibility, and a variable concentration of the incorporated dye, for allowing quasi-infinite number of luminescent centers. Typical key-issues for quantum dots as well as metal-doped nanoparticles, such as high-temperature crystallization and core-shell type surface conditioning, do not need any consideration, here. Metal-doped oxides materials (e.g. LaPO4:Ce,Tb, CaF2:Ce,Tb, YVO4:Eu or Zn2SiO4:Mn) that we have also proposed for incorporation into MOFs by nature comprise several of the above listed disadvantages, too. |
|
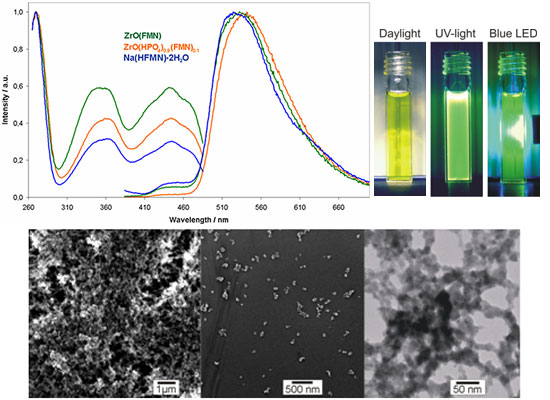 ZrO(FMN) – a novel concept of organic-inorganic hybrid luminescent nanomaterials. |
|
While introducing the concept of dye-modified zirconium phosphates (DMZP’s) as an alternative class of luminescent nanomaterials, it turned out that these nanomaterials allow for controlled switching of the luminescence. Thus, the green emission of the system ZrO(HPO4)1-x(FMN)x can be reversibly turned on and off. In concrete, the green emission is switched off by reduction, such as in the presence of [S2O4]2-, N2H4 or H2. The emission is switched on by re-oxidation, such as in the presence of O2. Moreover, the emission of the inorganic-organic hybrid nanomaterials can be modified by introducing alternative fluorescent dyes. Thus, the luminescent nanomaterial ZrO(UFP) (UFP: umbelliferonephosphate) shows emission of blue light. First experiments evidence that an incorporation of ZrO(FMN) nanoparticles into MOFs is possible. Next steps will address additional emission colors of the nanoparticles, the incorporation of luminescent nanoparticles in MOFs as well as the controlled switching of the light emission. |
|
Exohedrally functionalized fullerene derivatives are investigated regarding their potential as connectivity centers in novel inverse MOFs. This offers two perspectives: Intrinsic luminescence with improved quenching options upon sorption as well as the inclusion of luminescent nanoparticles into the inverse MOF. Functionalized fullerenes offer nanometer spacing from their own extensions in contrast to conventional MOFs and ZIFs that do not incorporate these particles. |
|
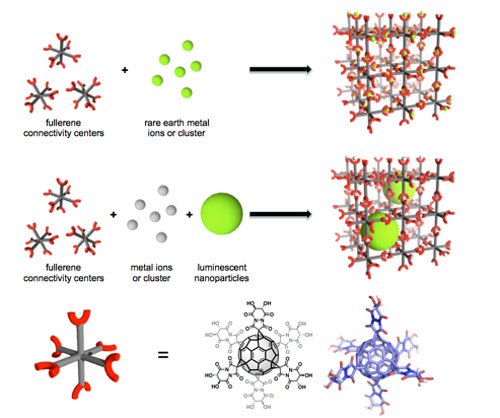 |
Publications: |
P.R. Matthes, C. J. Höller, M. Mai, J. Heck, S. J. Sedlmaier, S. Schmiechen, C. Feldmann, W. Schnick, K. Müller-Buschbaum |
|
A. Zurawski, J.-C. Rybak, L. V. Meyer, P. R. Matthes, V. Stepanenko, N. Dannenbauer, F. Würthner, K. Müller-Buschbaum |
|
A. Zurawski, M. Mai, D. Baumann, C. Feldmann, K. Müller-Buschbaum |
|
M. Roming, C. Feldmann |
|
J.-C. Rybak, C. J. Höller, P. R. Matthes, K. Müller-Buschbaum, M. Mai, C. Feldmann, R. Köhn |
|
J.-C. Rybak, I. Schellenberg, R. Pöttgen, K. Müller-Buschbaum |
|
A. Zurawski, F. Hintze, K. Müller-Buschbaum |
|
C. J. Höller, K. Müller-Buschbaum |
|
C. J. Höller, M. Mai, C. Feldmann, K. Müller-Buschbaum |
|
C. J. Höller, P. Matthes, J. Beckmann, K. Müller-Buschbaum |
|
J.-C. Rybak, M. Tegel, D. Johrendt and K. Müller-Buschbaum |
|
M. Roming, H. Lünsdorf, K. E. J. Dittmar, C. Feldmann |
|
M. Roming, H. Lünsdorf, K. E. J. Dittmar, C. Feldmann |
|
H. Goesmann, C. Feldmann |
|
H. Goesmann, C. Feldmann |
|
M. Roming, C. Feldmann |
OTHER EVENTS
- Calendar of events TU Dresden
- 16.-17.09.2013 (Dresden, GERMANY)
International MOF Symposium 2013 - 08.-11.09.2014 (Leipzig, GERMANY)
FEZA2014 - 28.09.-01.10.2014 (Kobe, JAPAN)
MOF2014 - Starting on 18.04.2014 (Hamburg, GERMANY)
Crystals & Symmetry Course
PUBLICATION NEWS
- TU Dresden’s DUT-6 claimed by other scientists
- 10.05.2012
Themed Issue on Metal-Organic Frameworks now published - 05.12.2012
Deuterium from a quantum sieve
CONTACT
Project Assistant
![]()
![]()
Phone: +49 351 463-33632
Fax: +49 351 463-37287
Email:
sekretariat-ac1@mailbox.tu-dresden.de
Office:
![]()
Bergstraße 66,
Neubau Chemische Institute,
Zi. 462
Mail to:
![]()
TU Dresden
Fachrichtung Chemie
und Lebensmittelchemie
![]()
Professur für Anorganische Chemie I
![]()
01062 Dresden
Bulk mail to:
![]()
Fachrichtung Chemie
und Lebensmittelchemie
![]()
Professur für Anorganische Chemie I
![]()
Helmholtzstraße 10
01069 Dresden