Suche

PRIORITY PROGRAM
“Porous Metal-Organic
Frameworks”
 Coordinator & Contact
Coordinator & Contact
 Summary
Summary
 Projects and
Projects andParticipants - Part I
 Projects and
Projects andParticipants - Part II
EVENTS
 Topical Workshop 2009
Topical Workshop 2009MOF Modelling for PhD
students
 Kickoff meeting 2009
Kickoff meeting 2009
 Topical Workshop 2010
Topical Workshop 2010Adsorption and Diffusion
in MOFs for PhD
students
 MOF 2010 - Marseille
MOF 2010 - Marseille Meeting after MOF 2010 -
Meeting after MOF 2010 - Marseille
 Workshop "MOF Synthesis and
Workshop "MOF Synthesis andStructure London 2010"
 2. Assessment SPP 1362
2. Assessment SPP 1362 in Dresden 2011
 Topical Workshop "Catalysis"
Topical Workshop "Catalysis"for PhD students Stuttgart
2011
 International Symposium on
International Symposium on
Metal-Organic Frameworks
2011 in Dresden
 Workshop "MOFs for industrial
Workshop "MOFs for industrial
applications Bergamo 2011"
 Topical Workshop "MOF-Based
Topical Workshop "MOF-Based
Chemical Sensors" München
2012
 MOF Status Report Meeting
MOF Status Report Meeting
2012 - Dresden
 International MOF Symposium
International MOF Symposium
2013 - Dresden
LINKS
 Chair of Inorganic Chemistry I
Chair of Inorganic Chemistry I
Redox-Active Metal-Organic Frameworks: Novel Entatic State Catalysts?
Consortium: |
Professor Dr. Karsten Reuter, Garching
|
Professor Dr. Dirk Volkmer, Augsburg
|
|
Project: |
Redox-Active Metal-Organic Frameworks: Novel Entatic State Catalysts? |
Abstract: |
Porous, redox-active metal-organic frameworks (MOFs) shall be developed which are constructed from aromatic N-donor ligands (e.g. pyrazolate or triazolate moieties) and openshell 3d transition metal ions. |
Results: |
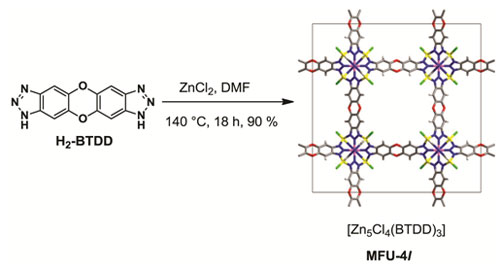
Figure 1. Synthesis of MFU-4l.
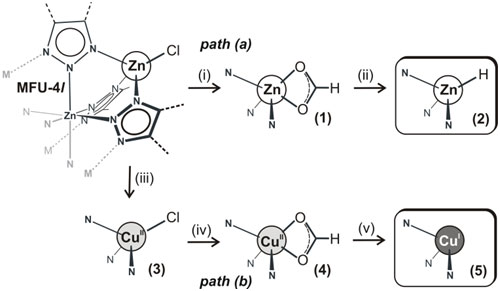
Figure 2. Preparation of MFU-4l derivatives with active metal sites. (i) HCOOLi/MeOH, RT; (ii) 300 °C, 30 min, in vacuum; (iii) CuCl2, DMA, 60 °C; (iv) HCOOLi/MeOH, RT, (v) 180 °C, 1 h, in vacuum |
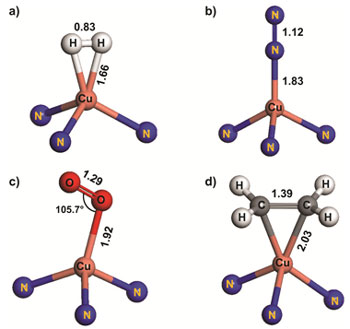
A novel highly porous member of isoreticular MFU-4-type frameworks, [Zn5Cl4(BTDD)3] (MFU-4l(arge)) {H2-BTDD = bis-(1H-1,2,3-triazolo-[4,5-b],[4’,5’-i])dibenzo-[1,4]-dioxin}, has been synthesized using ZnCl2 and H2-BTDD in N,N-dimethylformamide as a solvent. MFU-4l represents the first example of MFU-4-type frameworks featuring large pore apertures of 9.1 Å. TGA and variable temperature XRPD experiments carried out on MFU-4l indicate that it is stable up to 500°C (N2 atmosphere) and up to 350 °C in air. Heating up a MFU-4l suspension with MCl2 solutions in DMF or DMA leads to isostructural replacement of zinc by M(II) centres, where M = Mn, Fe, Co, Ni or Cu. The complete exchange of tetrahedrally coordinated zinc centers can only be achieved with CoCl2 and requires a huge excess of cobalt ions in the suspension (Co/Zn molar ratio > 5). The central octahedrally coordinated Zn center cannot be replaced at the given experimental conditions. Thus, the average chemical formula of obtained frameworks may be formulated as [Zno(MxtZn(4-x)tCl4(BTDD)3]. Postsynthetic metal-exchange in MFU-4l can be augmented by ligand exchange reactions (as exemplified for chloride/formate exchange in MFU-4l and Cu-MFU-4l) leading to a variety of frameworks with different redox active 3d transition metal ions. Upon thermal treatment of MFU-4l formates, coordinatively strongly unsaturated metal centers, such as Zn(II)-hydride or copper(I) species, are generated selectively. Cu(I)-MFU-4l, prepared in this way, is stable at ambient conditions and shows fully reversible chemisorption of small molecules such as O2, N2 and H2 with corresponding isosteric heats of adsorption of 53, 42 and 32 kJ mol-1, respectively, as determined by gas sorption measurements and confirmed by density-functional theory calculations (Table 1). Moreover, Cu(I)-MFU-4l forms stable complexes with C2H4 and CO, which have been characterized by FT-IR spectroscopy. The corresponding stretch modes of coordinatively bound CO and C2H4 molecules can be observed in the FT-IR spectra at 2081 cm-1 and 1541 cm-1, respectively and are shifted to lower wavenumbers, as compared to free gas molecules (2143 cm-1 for CO and 1623 cm-1 for C2H4). In all cases, a 1:1 stoichiometry of CuI-O2 (N2, H2, C2H4 or CO) coordination units has been observed. The chemisorption of O2, N2, H2 and C2H4 is fully reversible at ambient conditions, whereas CO can only be desorbed at 270 °C. MFU-4l hydride derivative 2 reacts with benzoyl chloride in benzene solution at RT, yielding benzaldehyde as the main product. |
| Table 1. Experimental and DFT-calculated isosteric heats of adsorption in kJ mol-1 in Cu(I)-MFU-4l |
| H2 | N2 | O2 | C2H4 | ||
| Experiment | 32.3±0.4 | 41.6±0.6 | 52.6±0.6 | 88±4 | |
| DFT-B3LYP | 25 | 44 | 46 | 84 |
|
Figure 3. Binding geometries for H2 (a), N2 (b), O2 (c) and C2H4 (d) at the Cu(I) sites within the Kuratowski unit of MFU-4l as obtained from DFT calculations (atomic distances in Å). |
The demonstrated hydride transfer on electrophiles and strong binding of small gas molecules suggests these novel – yet robust – metal-organic frameworks with open metal sites as promising catalytic materials comprising earth-abundant metal elements. A density-functional theory (DFT) based screening of MFU-4l frameworks with different metal centers and side ligands, suitable for O2 activation, has been performed in order to support the synthesis endeavours (FHI - aims code: PBE vs. PBE0 functional (with vdW corrections)). As a model system, instead of full periodic MFU-4l, Kuratowski and Scorpionate complexes have been used. PBE systematically gives larger binding energies if compared to PBE0. Detailed analyses of Co-complex show that the major error here is not in the O2/complex interaction but rather in the re-hybridization of cobalt orbitals in the ligand field (largely independent of the specific ligand). A larger crystal field splitting in PBE0 creates a larger “cost” for rehybridization to octahedral configuration, which in turn implies the much smaller binding energy. |
| Co-Kuratowski komplex |
| Eb(O2)(eV) | Cl-1 | F-1 | H-1 | CN-1 | OH-1 | NH2-1 | NO2-1 | |
| PBE | -0.58 | -0.84 | -1.99 | -1.08 | -1.02 | -1.29 | -1.01 | |
| PBE0 | 0.9 | 0.57 | -0.44 | 0.38 | 0.43 | 0.13 | 0.54 |
Publications: |
D. Denysenko, M. Grzywa, M. Tonigold, B. Streppel, I. Krkljus, M. Hirscher, E. Mugnaioli, U. Kolb, J. Hanss, D. Volkmer
|
|
M. Tonigold, Y. Lu, A. Mavrandonakis, A. Puls, R. Staudt, J. Möllmer, J. Sauer, D. Volkmer
|
|
D. Denysenko, T. Werner, M. Grzywa, A. Puls, V. Hagen, G. Eickerling, J. Jelic, K. Reuter, D. Volkmer
|
|
Y.-Y. Liu, K. Leus, M. Grzywa, D. Weinberger, K. Strubbe, H. Vrielinck, R. Van Deun, D. Volkmer, V. Van Speybroeck, P. Van Der Voort
|
|
S. Biswas, S. Couck, M. Grzywa, J. F. M. Denayer, D. Volkmer, P. Van Der Voort
|
|
M. Grzywa, D. Denysenko, J. Hanss, E.-W. Scheidt, W. Scherer, M. Weil, D. Volkmer
|
|
A. S. Dorcheh, D. Denysenko, D. Volkmer, W. Donner, M. Hirscher
|
|
A. Soleimani-Dorcheh, R. E. Dinnebier, A. Kuc, O. Magdysyuk, F. Adams, D. Denysenko, T. Heine, D. Volkmer, W. Donner, M. Hirscher
|
|
J. Teufel, H. Oh, M. Hirscher, M. Wahiduzzaman, L. Zhechkov, A. Kuc, T. Heine, D. Denysenko, D. Volkmer
|
|
M. Grzywa, C. Gessner, D. Denysenko, B. Bredenkötter, F. Gschwind, K. Fromm, W. Nitek, E. Klemm, D. Volkmer
|
|
P. Schmieder, D. Denysenko, M. Grzywa, B. Baumgärtner, I. Senkovska, S. Kaskel, G. Sastre, L. van Wüllen, D. Volkmer
|
|
M. Grzywa, B. Bredenkötter, D. Denysenko, S. Spirkl, N. Wojciech, D. Volkmer
|
|
J. Jelic, D. Denysenko, D. Volkmer, K. Reuter
|
|
P. Sippel, D. Denysenko, A. Loidl, P. Lunkenheimer, G. Sastre, D. Volkmer
|
|
D. Denysenko, M. Grzywa, J. Jelic, K. Reuter, D. Volkmer
|
|
G. Sastre, J. van den Bergh, F. Kapteijn, D. Denysenko, D. Volkmer
|
OTHER EVENTS
- Calendar of events TU Dresden
- 16.-17.09.2013 (Dresden, GERMANY)
International MOF Symposium 2013 - 08.-11.09.2014 (Leipzig, GERMANY)
FEZA2014 - 28.09.-01.10.2014 (Kobe, JAPAN)
MOF2014 - Starting on 18.04.2014 (Hamburg, GERMANY)
Crystals & Symmetry Course
PUBLICATION NEWS
- TU Dresden’s DUT-6 claimed by other scientists
- 10.05.2012
Themed Issue on Metal-Organic Frameworks now published - 05.12.2012
Deuterium from a quantum sieve
CONTACT
Project Assistant
![]()
![]()
Phone: +49 351 463-33632
Fax: +49 351 463-37287
Email:
sekretariat-ac1@mailbox.tu-dresden.de
Office:
![]()
Bergstraße 66,
Neubau Chemische Institute,
Zi. 462
Mail to:
![]()
TU Dresden
Fachrichtung Chemie
und Lebensmittelchemie
![]()
Professur für Anorganische Chemie I
![]()
01062 Dresden
Bulk mail to:
![]()
Fachrichtung Chemie
und Lebensmittelchemie
![]()
Professur für Anorganische Chemie I
![]()
Helmholtzstraße 10
01069 Dresden